By Dr Minh Alexander retired consultant psychiatrist 5 June 2023
Today Croydon Employment Tribunal is scheduled to hear a whistleblowing doctor’s claim against University Hospitals Sussex NHS Foundation Trust. The hearing is set to run until 16 June 2023.
This is a post to share a relevant report from a 2020 Health Education England review of core and higher surgical trainees’ experience at the trust, carried out after trainees raised concerns:
Urgent Concern Review (On-site visit) Core and Higher General Surgery, March 2020
Trainees valued the supervision of some consultants but otherwise raised many safety and governance issues with the HEE reviewers. These included:
- An “unpleasant”, “toxic” atmosphere
- Poor behaviour by some consultants
- “Trainees informed the review team of examples of bullying and undermining by a named consultant, towards both other consultants and trainees.”
- “The review team heard that some consultants frequently demonstrated undermining behaviour at the morning handover for the upper and lower gastrointestinal (GI) team, for example criticism of the registrars’ management of patients and arguments between themselves.”
- “Trainees described a recent incident where the named consultant had undermined a consultant colleague in a large meeting by repeatedly accusing them of being incapable of performing a basic surgical procedure.”
- High complication rates and poor recording of clinical outcomes“
- “The review team heard of a case in which a patient had received surgery on the wrong part of their bowel, resulting in recurrent readmissions.”
- Trainees expressed concern at the apparent increase in death rates over a period of years, to levels which they felt were unexpected considering the demographics of the local population. Trainees reported they had requested this was investigated by the Chief Medical Officer. When asked, trainees confirmed that the Medical Director and Chief Executive had been made aware of these concerns.” [my emphasis] At the time of the review, the trust Chief Executive was Marianne Griffiths.
- Lack of leadership in the department
- “….a lack of ownership of emergency surgery patients.”
- “The review team heard that some consultants frequently refused to review patients who were not going to theatre that day, resulting in trainees feeling unsupported when asking for senior advice on managing patients. The review team were informed that core trainees were often expected to act at a registrar level in terms of decision- making.”
- Poor quality clinical work by locum doctors which was not well supervised
- “Trainees reported a lack of confidence in the supervision provided by some locum consultants and provided examples related to clinical decision-making and dismissal of core trainees’ concerns about an unwell patient.”
- “The review team were informed there were three gaps (two non-training and one training) on the registrar rota which are currently unfilled. Trainees reported consultants were aware of concerns about the capability of some locum registrars who had covered the rota but had responded that they were required to fill the gaps.”
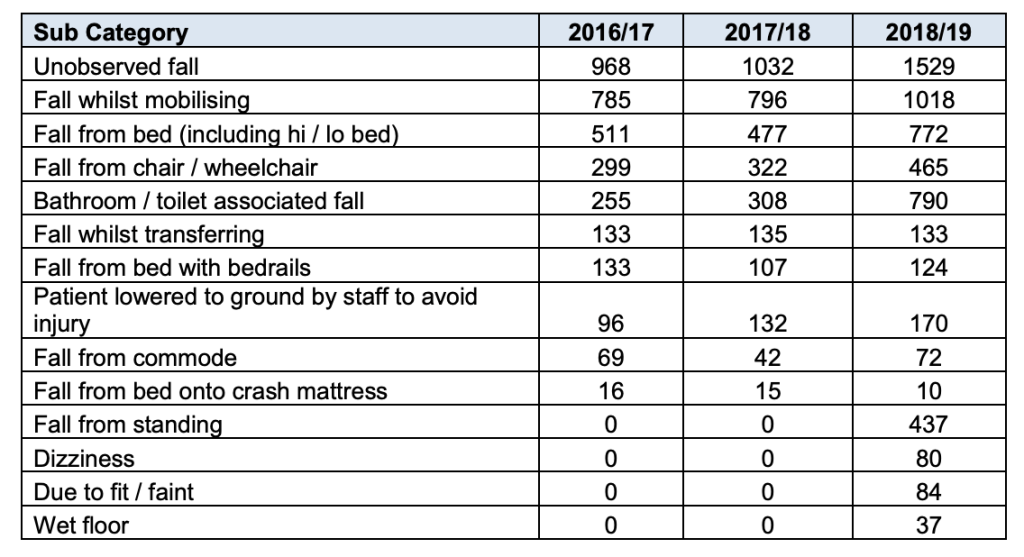
- “…..the review team were informed of a review of the last 100 elective colorectal resections performed (taking place between September 2019 and 3 February 2020), which indicated that the anastomotic leak rate was between 10.5-11.5% depending on the definition used to classify as a leak. The review team also noted of the cases reviewed: 14% returned to theatre, 12% experienced wound infections and 15% required readmission. Trainees reported they would not want a family member operated on by some of the consultants in the department.” [my emphasis]
- “Trainees reported complications were inadequately discussed at morbidity and mortality (M&M) meetings due to the volume of complications.”
- “The review team heard an example of a serious incident being closed down without sufficient review.”
- “The review team heard that results of an audit showing that M&M does not meet Royal College of Surgeons guidelines had been shared with the Clinical Lead for General Surgery. Trainees reported they had also raised concern at the Local Faculty Group (LFG) around the accuracy of M&M data and received the response that all departments were struggling with this. The review team were concerned to hear of the lack of systematic governance of M&M data collection. Furthermore, the review team were informed that M&M meetings were not well attended by consultants and until recently minutes of the meetings had not been recorded.”
- Trainees’ hours were not appropriately controlled and in some cases exception reporting had been discouraged.
- “The review team heard that trainees regularly worked additional hours. When asked, trainees reported they did not submit exception reports because some consultants had discouraged F1 doctors from doing so. The review team were informed that the Guardian of Safe Working Hours had recognised a drop in exception reporting and approached a higher trainee regarding this. Trainees reported a lack of leadership and responsibility around exception reporting.”
Of great concern, the HEE reviewers were told that trainees were discouraged from raising concerns, including with HEE.
A senior manager was reportedly one of the individuals who had discouraged the raising of concerns:
“The review team were particularly concerned to hear of behaviours apparently intended to discourage trainees from raising concerns. The review team were informed that a consultant had sent an email to trainees ahead of the review, appearing to put pressure on trainees to provide positive feedback. Furthermore, the review team heard that a trainee had been told by a senior manager that those who raised concerns needed to appreciate the consequences of their actions in terms of the impact on service provision.”
The HEE reviewers clearly believed and took what the trainees reported seriously, as reflected in their list of mandatory requirements to the trust.
These included requirements for improved governance and resolution of the cultural issues:
“HEE require the Trust to develop robust clinical governance processes, in line with NHS England and NHS Improvement (NHSE/I) requirements, in relation to patient safety, complication rates and record keeping with regards to patient outcomes.”
“HEE require the Trust to carry out work to improve the culture and reduce the number of bullying and undermining incidents within the general surgical department.”
As part of the action plan on bullying, HEE required: “the Trust to consider an external review of the department from the Royal College of Surgeons.”
I understand that this RCS external review took place only last month, three years on from the original recommendation. I am asking the trust to confirm that this is so, and about progress in general on HEE’s requirements.
The National Guardian and CQC at Sussex
So in summary, HEE found very serious dysfunction in a major trust department, with substantial patient safety implications and reports of actual care failings.
Concerns about the death rates had been raised with Marianne Griffiths.
HEE also found evidence of suppression, including by a senior manager.
And guess who carried out a review of trust whistleblowing governance in July 2019, eight months before the damning findings by HEE in March 2020?
The former NHS National Freedom To Speak Up Guardian, Henrietta Hughes.
Hughes received concerns from trust staff in December 2017, but ignored her own policy and procedure, and astonishingly postponed a review until 2019 to give the trust time to improve.
Her July 2019 review predictably praised the trust leadership for making improvements, and reflected none of the dysfunction found by HEE the following Spring.
CQC would have been informed of the Spring 2020 HEE review findings as part of multi-agency protocols. There is no record of a CQC inspection of trust surgical services in response to the concerns.
An unannounced Care Quality Commission inspection of several trust sites in September/October 2021 took place after staff whistleblew. CQC found care failings and poor whistleblowing governance.
Yet Griffiths and co were still protected and CQC let them keep their “Outstanding” overall rating.
But the CQC continued to hear from trust whistleblowers:
“CQC then received concerns about the UGI surgical service from staff and other stakeholders. We carried out an inspection of the elective UGI surgical service in August 2022 and found serious safety and leadership concerns.”
“We have continued to receive concerns from staff about the safety of the surgical services at the Royal Sussex County Hospital.”
Finally, last month, even the CQC conceded that the trust was failing and downgraded it from “Outstanding” to “Requires Improvement” overall, and “Inadequate” on the Well Led domain. In its inspection report, CQC noted:
“Some staff feared reprisal for raising concerns and others had simply given up because of ‘concern fatigue.’ This group of staff felt there was little point raising concerns because no action was taken when they did. When we asked staff to describe the culture of the trust, the feedback was mostly negative. Staff also felt the trust was a ‘hierarchical’ organisation which made it hard to get their voice heard.’
CQC also admitted in its briefing, upon release of its report:
“We continue to have repeated contact from staff who tell us feel unable to raise concerns through the trust’s own internal escalation processes.”
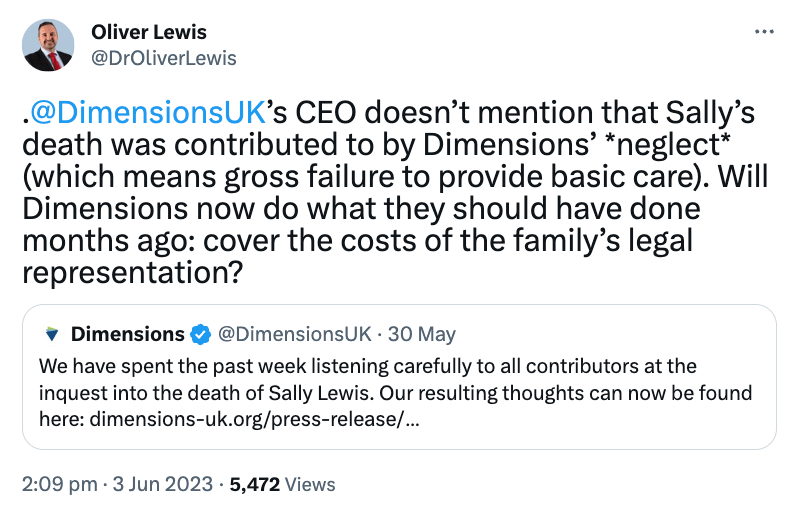
How many Sussex staff and patients suffered because Henrietta Hughes gave Marianne Griffiths et al a free pass in 2018?
Or because the CQC failed to act quickly enough on concerns, including those raised by HEE in 2020?
What is Hughes doing as the Patient Safety Commissioner?
And importantly, what do HEE’s findings of serious governance failings and suppression, CQC’s similar findings, and Sussex’s apparent failure to organise a timely external review of surgical services as advised by HEE – despite Griffiths having already been informed of rising death rates – say about Marianne Griffiths’ current investigation of North East Ambulance Service for NHS England?
UPDATE 10 JUNE 2023
Matters took a dramatic turn at the opening of the Sussex whistleblowing ET case on 5 June 2023 when the police asked to sit on evidence, because it was relevant to a criminal investigation of gross negligence manslaughter at Sussex. The trust subsequently asked for an adjournment on grounds of prejudice to their witnesses. But it means that the skeletons in Griffiths’ (and her former medical director now successor CEO) cupboard have tumbled out:
Police investigate dozens of deaths at hospital in Brighton
Alongside this, NHS England continues to resist questions, raised over the last year, regarding the legitimacy of the investigation of NEAS headed by Marianne Griffiths. In its most recent letter, NHSE took the bizarre and desperate step of pretending that it had no jurisdiction over NHS Foundation trust governance, in order to avoid addressing the issue:
UPDATE 11 JUNE 2023
I have asked the CQC, which employs the National Guardian, to investigate its handling of the whistleblowing matters at Sussex:
A little more on the dramatis personae
Henrietta Hughes got an OBE, and here is Marianne Griffiths congratulating her about it:
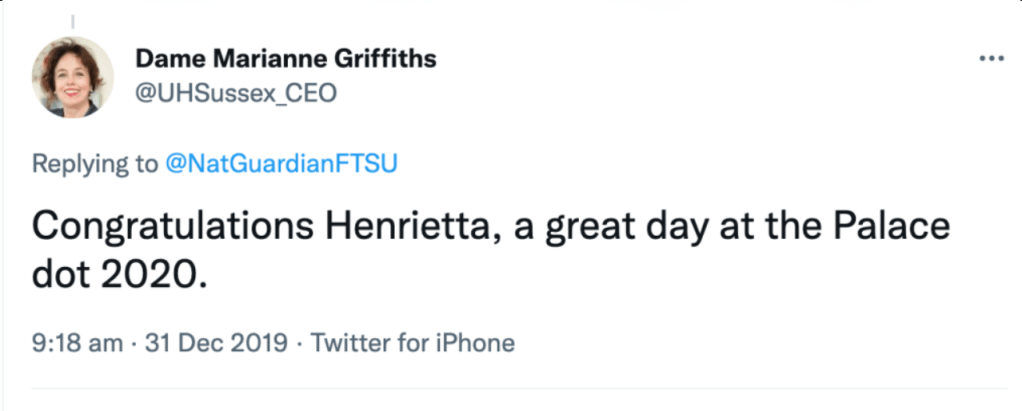
Griffiths was made a dame in the 2018 New Year’s honours list, eight months after Hughes gave her the free pass.
George Findlay the former Sussex medical director, promoted to trust CEO, has opined on how much things have improved since the last CQC inspection:
NHS boss sets out how hospital trust has improved after damning report.
Findlay told the media the improvements included:
“Trust-wide focus on making it easier for people to speak up and raise concerns, including stronger support for the Freedom To Speak Up service. Results from the anonymous monthly “Pulse” staff survey show more people now feel confident that the trust would act upon concerns that were raised, up from 49 per cent in September 2022 to 58 per cent in March 2023, closing in on the best-performing trusts nationally.”
Having 42% staff who do not believe concerns will be acted upon is not great cause for celebration. And that is if one accepts that the trust’s report / its pulse surveys are reliable. It is hard to imagine that any patients with botched surgery, leaky bowels, post operative infections and readmissions, or bereaved families, would be uncorking any champagne.

In April 2021, shortly before he was promoted to CEO, Findlay had these commendable aspirations on quality for patients and staff, as expressed in a video posted on Facebook by the trust:
“Welcome to University Hospitals of Sussex. My name is Dr George Findlay. I am Chief Medical Officer and Deputy Chief Executive. We’re absolutely passionate about providing the highest quality care for patients every time and the best environment for our staff to work in. We’re really clear about the continued investment in services and we’ll continue to provide accident and emergency services, maternity services, trauma services and special services in all the locations we currently do. We’re absolutely passionate about research and innovation and working with our partner universities. We’ll make sure that we adopt best practice, our best roles and make the best value of evidence for our patients for the future.”
RELATED ITEMS
University Hospitals Sussex NHS Foundation Trust resisted FOI requests about their current whistleblowing claims: