Dr Minh Alexander retired consultant psychiatrist
University Hospitals Sussex NHS Foundation, the subject of a police investigation, is resisting an FOI request.
The wheels came off the trust’s publicity machine earlier this year when the CQC finally conceded that there was poor trust leadership and downgraded it sharply from ‘Outstanding’ to ‘Requires Improvement’ overall, with a rating of ‘Inadequate’ on leadership:
Sussex hospital trust downgraded as staff blow whistle
But even before that, the CQC had registered failures of leadership and very poor whistleblowing governance.
Whistleblower controversies have dogged the trust, as have allegations of spin and suppression by its senior management team, who were for a while protected favourites of Jeremy Hunt.
As the forcefield of protection has weakened, serious failings have become evident.
One matter of concern is how exactly a predecessor body was lifted out of special measures in 2019, and there have been questions about the quality of the information which informed this regulatory decision.
Accordingly, I asked the trust for all reports undertaken by the controversial Good Governance Institute for the trust, some of which informed this regulatory decision.
This is especially as one of the GGI consultants, Darren Grayson, was later appointed as the trust’s Chief Governance Officer in March 2022.
Darren Grayson was a GGI Executive Director and Partner in the period in which the trust purchased many services from the GGI, having previously resigned as CEO of a neighbouring NHS trust in 2015. The resignation followed a very critical Care Quality Commission report which concluded there was bullying by management. The unions and MPs demanded new leadership.
University Hospitals Sussex NHS Foundation Trust has responded oddly to my FOI request for the GGI reports. At first it resisted disclosure of all the requested GGI reports. The trust also claimed it could not disclose the cost of its contracts with the GGI on grounds of commercial interest, which is nonsense because as a public body it is expected to be proactively transparent about spending and to publish details of spending over £25K.
For unexplained reasons, the trust has now had a change of heart and disclosed some of the GGI reports but not a review of a clinical service, and two other reports on governance.
I cannot find any given trust grounds for withholding the latter two governance reports.
Moreover, the conduct of a clinical service review by the GGI is unusual. I am not aware that the GGI normally reviews clinical services. Indeed, most NHS trusts call in the Royal Colleges for reviews of clinical services so that they can be informed by appropriate clinical expertise.
The trust contends that disclosure of the clinical service review report is “likely” to lead to “sensationalised” reporting. The trust is so reluctant to disclose this particular report that it has claimed that if it has to disclose this report, it might not carry out invited reviews again:
“Disclosure at this stage would likely to prejudice the Trust’s ability to carry out its public affairs effectively…This would likely undermine its [the trust’s] willingness to invite external organisations to conduct service reviews in the future.”
The so far disclosed GGI reports are as follows:
Quality Governance Review at BSUH by Good Governance Review November 2017
I leave it with the reader to decide if these reports represent public money well spent. They are generally upbeat in tone.
The fact that serial reports were carried out – there were a total of seven GGI reports at Sussex – brings to mind observations by Professor Sturdy, University Bristol, to BBC Newsnight about demand inflation by external consultants, who want repeat business.
This is the trust’s first FOI response letter of 12 July 2023.
This is the trust’s second FOI response letter of 10 August 2023.
The trust also decided that it would disclose spending on the GGI after all. It amounts to £410,419.99 in the period November 2017 to November 2020.
I believe that the 2018 clinical service review that is still being withheld is of significant public interest, and forms part of the audit trail on corporate failures. It was co-authored by Darren Grayson, now the trust’s Chief Governance Officer.
The trust has stated that Dr George Findlay the trust’s former medical director, now CEO, is the trust’s qualified person under FOIA who has agreed with the decision not to disclose. As the qualified person he is ultimately responsible for the decision to withhold this important report.
I have challenged the trust’s claimed grounds for withholding the report of the clinical service review in the following, hopefully self-explanatory correspondence to its Chair.
BY EMAIL
Alan McCarthy
Chair University Hospitals Sussex NHS Foundation Trust
13 August 2023
Dear Mr McCarthy,
Decision by George Findlay to withhold a 2018 GGI report on a Rapid Review of Digestive Diseases Clinical Directorate.
I write to request an internal review of a decision by Dr Findlay trust CEO and former trust medical director to withhold information under FOIA which I consider should be disclosed in the public interest.
Please see the attached FOI correspondence from the trust on these matters.
Firstly, I am concerned irregularity in the trust’s FOI process.
The trust withheld several GGI reports from me which it later disclosed.
The trust also initially claimed quite inappropriately that it could not disclose the cost of each piece of work that it contracted from the GGI on grounds of commercial interest, when in fact that exemption did not apply, and in any case the trust should be proactively publishing all items of spending above £25K.
The trust has now disclosed four of seven of the reports that the GGI has produced. The seven GGI reports were as follows:
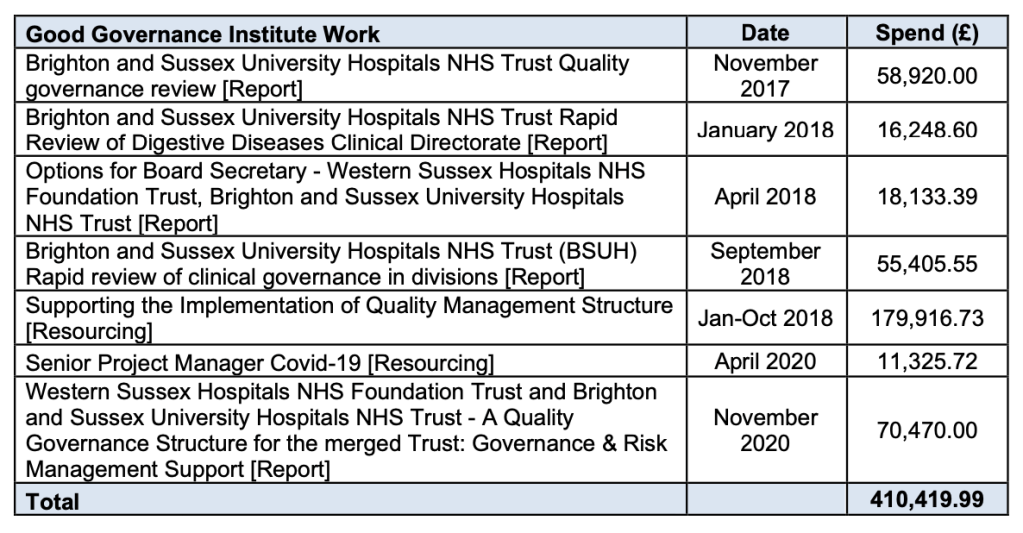
But the trust has not released the two reports on:
– “Supporting the Implementation of Quality Management Structure” 2018
– “Senior Project Manager Covid-19” 2020.
I could not find any grounds given in the trust’s FOI response letter for not disclosing these two reports.
I request that the trust either discloses the reports or provides valid legal exemptions for withholding them. I also ask if Darren Grayson was a GGI consultant for either of these exercises and whether the “Supporting the Implementation of Quality Management Structure” report made any recommendations for the creation of a Chief Governance Officer post or similar.
Of special concern, the trust declined to disclose the 2018 Brighton and Sussex University Hospitals NHS Trust Rapid Review of Digestive Diseases Clinical Directorate.
The trust has claimed FOIA Section 36 exemption, prejudice to the conduct of public affairs against disclosure of this report.
I copy below in the appendix the lengthy justification that the trust has provided for claiming this exemption.
The trust has disclosed that the ultimate decision maker in withholding the rapid review report on the Digestive Diseases Clinical Directorate is Dr George Findlay trust medical director:
“This decision is supported by the Trust’s Qualified Person, Dr George Findlay (Chief Executive Officer), who is of the opinion that the disclosure of this information would likely inhibit the free and frank provision of advice and/or free and frank exchange of views for the purposes of deliberation [section 36(2)(b)(i) and (ii)], and that disclosure would otherwise likely prejudice the effective conduct of public affairs [section 36(2)(c)].”
The GGI has redacted the names of all GGI report authors, claiming FOIA S40 personal data and S43 Commercial interest exemptions.
“Although disclosure in this case would serve a legitimate interest relevant to the general principles of transparency and accountability, it is our view that these individuals would not have a reasonable expectation that their personal information would be disclosed into the public domain in this way. On this basis we do not consider disclosure necessary nor do we consider there to be sufficient legitimate interest which outweighs the rights of the data subjects in this case. The redacted information is therefore considered exempt under section 40(2) [personal information] exemption of the Act. The engagement of s.40(2) in this case is considered absolute and is not subject to further public interest considerations.
Since our initial response on 12 July 2023, the Trust further considered the public interest test associated with section 43(2) [commercial interests] exemption as it relates to the disclosure of these reports and concluded that disclosure outweighed withholding this information. No information has been redacted under section 43(2) exemption within these reports.”
My challenge to the trust’s decision to withhold the report of the 2018 rapid review of the Digestive Diseases Clinical Directorate is as follows:
I reject the trust’s claim that the need to protect internal decision making and deliberations outweighs the public interest need to disclose.
Indeed, I consider that this argument suggests a lack of insight by senior trust management and a failure to create a genuinely safe space in which concerns can be openly acknowledged.
Trust staff have clearly felt unable to raise concerns without reprisal as evidenced by recurrent Care Quality Commission inspection findings, Health Education England inspection findings, disclosures by staff to the media and the ongoing legal claims against the trust for whistleblower detriment.
I believe the withheld rapid review report on the Digestive Diseases Clinical Directorate is in fact part of the evidence trail on corporate failures to protect patient safety and ensure proper processing of staff concerns.
Contrary to the trust’s claims, all trust staff who have continued to feel suppressed and silenced by trust management since 2018 would surely welcome publication of this report.
This is especially so as corporate failure is a theme that has specifically been raised by trust whistleblowers and their concerns have triggered a police investigation into deaths where the allegations may amount to gross negligence manslaughter.
I consider the report’s contents to be of very serious public interest and that it is indefensible and unsustainable that the trust claims publication would inhibit full and frank internal discussion.
There is currently no evidence of full and frank internal discussion and safe exchanges between frontline staff and senior management at the trust, and it is a flawed argument to claim that more suppression will improve the culture.
The trust speciously contends that because the GGI’s 2018 report was produced on the basis of an invited review, and it was not a regulatory report, this lowers the expectations of public disclosure:
“Since this was an invited review and not a regulatory investigation, the willingness of staff to continue to participate and cooperate in this process is essential. Full disclosure would be likely to discourage the staff that participated in the review and those in the wider department, from continuing to assist the Trust. This would likely hinder the Trust’s ability to develop its corporate improvement plan and implement the changes that are required. Disclosure at this stage would be premature and therefore likely to prejudice the outcome of the review process.”
I reject this argument because the report was purchased with £16,248.60 of public money, and there should be accountability and transparency about what was purchased. I also reject the trust’s claim of a lesser duty to disclose because it was only an invited review. This is not least because it has been widely acknowledged that there has been a general failure of NHS accountability in not publishing invited reviews, which has impeded system learning, facilitated cover ups and represents an abuse of the public purse. BBC Panorama exposed the fact that at least 111 invited reviews had been suppressed and it led to questions in parliament and demands for new procedures to require publication.
The trust’s claim that it would be dissuaded from commissioning future invited reviews if it is forced to disclose the 2018 GGI rapid review of the Digestive Diseases Clinical Directorate only reinforces the impression that the NHS has a tendency to consider invited reviews, paid for from the public purse, as private property:
“Disclosure at this stage would likely to prejudice the Trust’s ability to carry out its public affairs effectively…This would likely undermine its [the trust’s] willingness to invite external organisations to conduct service reviews in the future.”
The trust states that it would prefer to update the public with a report about improvement through upcoming board meetings. I reject this as a reason for not disclosing the GGI report. The public has a right to see the details of the original baseline against which all claims of improvement will be made. Without the baseline information, the public would not be truly free to make up their own minds about what improvement has been made. The trust is a public body with duties of accountability. Its board meetings must be genuinely accountable and disclose all relevant information, so that the public can ask fully informed questions. The board should not cherry pick or filter information to give a favourable impression. Reputation management is not a valid FOIA exemption.
Moreover, five years have passed since the rapid review of the Digestive Diseases Clinical Directorate. Expectations of improvement are greater given the time elapsed, and there is a greater burden on the trust to demonstrate clearly the level of improvement. The trust’s arguments that it needs time to make improvements are unreasonable given that five years have elapsed.
The trust has taken me to an ICO ruling which concluded that as only two months had elapsed after an invited review, an NHS trust should be given time to compose itself and respond before disclosing the contents of the review.
However, at Sussex, if after five years the trust has not put sufficient blue water between its practice now compared to its practice in 2018, the argument for disclosure is even greater because of questions about failure of improvement. If the trust has duly improved, there should be no good reason to suppress the 2018 report.
Moreover, as the trust made claims of substantial improvement and dismissed or minimised many staff concerns about clinical safety, it is of concern that the trust now relies on this argument to justify withholding the rapid review report:
“Although we appreciate that considerable time has passed since the review, some of the more complex issues involved remain live. The information that staff provided remains relevant to the status of the service at this time and issues are ongoing for the staff involved.” [my emphasis]
The trust cannot have it both ways.
I reject the trust’s claim that the rapid review report on the Digestive Diseases Clinical Directorate cannot be disclosed because disclosure would affect public confidence as follows:
“Disclosure at this time would also likely negatively impact the relationship the Trust has with staff involved in this process, thereby hindering its ability to implement the changes that are required. This would in turn compromise the Trust’s ability to assure the public that it is taking the necessary action to improve patient care.”
I contend that this is a specious and speculative argument which is not about the public interest but about reputation management. This same argument is not applied when there are adverse regulatory findings. Instead, it usually acknowledged that there must first be accountability and transparency about difficulties, before those difficulties can be addressed. The argument presented by the trust raises a question about whether the trust is clutching at straws to protect senior reputations, and to retrospectively defend information that was given to regulators in 2018 to justify lifting the trust out of special measures. When perhaps there was cause to doubt the optimism expressed in the other GGI reports that have so far been disclosed.
I also note that the trust willingly placed adverse information in the public domain about the performance of the Digestive Diseases Clinical Directorate in 2016. This is an extract from a 2016 published trust board paper:
“Current Position
The operational standard is that 92% of patients who have not yet started treatment should be waiting no more than 18-weeks.
At BSUH, we are currently reporting 73% of patients waiting no more than 18-weeks. The backlog is now reducing with a total of 8,954 patients waiting over 18- weeks as of the 10 May. The deterioration in Digestive Diseases (Surgery and Medicine) has slowed down however they are unable to clear the current backlog with the current demand and capacity. Comprehensive plans are being developed to address the deterioration within Paediatrics especially ENT. Pain management continues to deteriorate and will require additional capacity in both admitted and non-admitted performance.
There are 100-patients that have waited over 52-weeks for treatment at the end of April. Of the 100-patients, 89 are within Digestive Diseases. 64- patients are awaiting surgery and the remaining 25-patients are awaiting investigations and follow-up appointments. The remaining 11-patients are across a range of specialties, but are all dated. The forecast is that only 18- patients will require dating in DD by July 2016.
There are now circa 5,000 patients transitioning through the NULL cohort of the Patients with Unknown Status (PUS). It will reduce further as systems and processes are improved and until such time is being routinely validated. The trust has commissioned an external IT specialist to review the current ‘business rules’ that generate our PTLs to ensure that we are reporting correctly and have sight of all patients.
Following the extensive validation of April month end, the external validation team focussed on validating the ‘planned outpatients’ who have had no target date assigned. This work was completed as the team finished. Work continues to validate the ‘to be checked’, ‘planned’ and ‘non-RTT’ cohorts of patients and ensure that we have full visibility of them, we are certain of the appropriateness of reporting and we have sufficient capacity to treat.”
It is inconsistent of the trust to have openly revealed these significant performance problems in the past, but to now claim that it cannot reveal adverse material in the 2018 GGI rapid review about the same clinical directorate.
The trust itself has created concern by claiming in its FOI response:
“Full disclosure would likely lead to greater speculation, external comment, media attention and/or pressure from other interested parties adding prejudice to the review process. Disclosure and subsequent use of this information by others without understanding the context in which it was written, is likely to lead to sensationalised or misunderstood reporting about the service in question.”
It is surely for the trust to provide context in the process of disclosure and to explain where it is appropriate and justifiable, what the disclosed data means.
Moreover, there is as yet no indication that the 2018 GGI Rapid Review of the Digestive Diseases Clinical Directorate was undertaken by anyone with relevant clinical expertise.
It is surprising that the trust did not ask the relevant Royal College to conduct an expert review of a clinical service and instead asked the GGI, an organisation which focuses on administrative matters. When medical Royal Colleges conduct invited reviews, these usually follow a very formal, standardised format and strict quality criteria, for important reasons of clinical quality, consistency and professional accountability to the public.
The trust disclosed in its previous response to me (attached) that Darren Grayson the trust’s current Chief Governance Officer was one of the GGI consultants who took part in the 2018 GGI’s Rapid Review of the Digestive Diseases Clinical Directorate.
I also understand that Mr Grayson is now acting as the senior trust liaison point for the police investigation into deaths at the trust.
In this context, I believe it is all the more important that the 2018 Rapid Review of the Digestive Diseases is disclosed so that there is transparency about any possible conflicts of interest in Mr Grayson directing the flow of evidence to the police.
Also, as you may be aware, BBC Newsnight recently investigated the Good Governance Institute and reported that the GGI has been calling itself an “Institute” without the express permission of the Secretary of State. Companies House advised Newsnight that this is an offence, and reportedly asked the GGI to stop calling itself an institute. There were also other concerns about the governance of the GGI.
In the light of this, it is additionally important that the trust discloses the GGI’s Rapid Review of the Digestive Diseases and demonstrates whether or not this review was conducted by appropriately qualified personnel, and to an acceptable standard. It would be a serious matter if a review of a clinical service and its safety was not conducted by anyone with the relevant clinical expertise and credentials.
I further note that the trust has spent a total of £410,419.99 on the Good Governance Institute’s services, in a period when Mr Grayson its current Chief Governance Office was a GGI director. According to Mr Grayson’s LinkedIn entry, he was a GGI Executive Director and Partner for the period September 2016 to March 2022.

The trust has taken me to an ICO ruling in favour of an NHS trust which pleaded against disclosure on grounds that staff might face hostility from the public if reviews of clinical services were disclosed. I ask the trust to consider the weight of ill health and unresolved grief on bereaved relatives who suffer from a lack of answers and gaslighting by NHS organisations who fail to be accountable and to take responsibility for failings.
Serious concerns were raised by the local Senior Coroner over several years about the trust’s patient safety record, with recurrent reports to Prevent Future Deaths sent to the Secretary of State and NHS regulators. It was also the current Coroner who triggered the ongoing police investigation by referring cases of concern. I ask you to acknowledge the serious suffering that is inflicted on surviving relatives, and the very great impact on their own health, when the truth is locked up and has to be dragged out. The NHS exists to serve, and not to be served.
Please therefore disclose the report of the 2018 GGI Rapid Review of the Digestive Diseases, with any relevant and proportionate redactions to protect individual staff privacy but without obscuring evidence of serious safety failings and any direct, indirect or implied evidence of corporate failures then or since. In particular, please also disclose:
- The trust’s terms of reference for this 2018 GGI rapid review
- The qualifications, professional credentials and seniority of all the GGI consultants who carried out the 2018 GGI Rapid Review of the Digestive Diseases and indicate whether they held any clinical qualifications or had relevant clinical experience.
Lastly, I believe there is a potential conflict of in that the trust’s “qualified person” under FOIA is Dr Findlay, who as the former medical director was directly involved in events regarding surgical safety, surgical competence and the employment case handling of medical whistleblowers at the trust.
I ask you to factor this into the trust’s further deliberations.
Lastly, I would like to ask some fresh questions as follows:
- Has the trust disclosed the 2018 GGI rapid review report of the Digestive Disease Clinical Directorate to the Care Quality Commission, and if so, when was the report shared with the CQC?
- If the trust presented any findings or summaries of the 2018 GGI Rapid Review of Digestive Disease Clinical Directorate at its board meetings, please provide copies of any relevant documents or excerpts from board minutes. (The published trust board papers on the trust’s website only extend back to 2021). Please also provide any updates that the trust has provided, through its trust board meetings, on improvement work flowing from the 2018 review of the Digestive Diseases Clinical Directorate. The ICO rulings to which the trust has directed me noted that other NHS organisations demonstrated good practice by issuing summaries in circumstances where they decided not to disclosure full reports.
- Has the report of the 2018 GGI Rapid Review report on the Digestive Diseases Clinical Directorate in fact already been disclosed to any external processes such as Employment Tribunal proceedings or other litigation?
Many thanks and best wishes,
Dr Minh Alexander
Cc
Caroline Lucas MP Brighton, Pavilion
BBC Newsnight
The Guardian
Dr Chaand Nagpaul
Dr Phillip Banfield BMA Chair
APPENDIX
Grounds given by the trust for not disclosing the 2018 report of the GGI’s Rapid Review of Digestive Diseases Clinical Directorate
“Brighton and Sussex University Hospitals NHS Trust Rapid Review of Digestive Diseases Clinical Directorate [Report] – January 2018
In December 2017, GGI was commissioned by Brighton and Sussex University Hospitals NHS Trust to conduct a rapid review of the Digestive Diseases Clinical Directorate; GGI’s review report is dated January 2018. This final report relevant to your request is being withheld in full on the basis that it falls within section 36(2) of the FOI Act [prejudice to the effective conduct of public affairs] and that the public interest in maintaining the exemption outweighs the public interest in disclosure. This decision is supported by the Trust’s Qualified Person, Dr George Findlay (Chief Executive Officer), who is of the opinion that the disclosure of this information would likely inhibit the free and frank provision of advice and/or free and frank exchange of views for the purposes of deliberation [section 36(2)(b)(i) and (ii)], and that disclosure would otherwise likely prejudice the effective conduct of public affairs [section 36(2)(c)].
Section 36 of the FOIA provides that, “Information to which this section applies is exempt information if, in the reasonable opinion of a qualified person, disclosure of the information under this Act – (2)(b) would, or would be likely to, inhibit –
i. the free and frank provision of advice, or
ii. the free and frank exchange of views for the purposes of deliberation,
or (2)(c) would otherwise prejudice, or would be likely otherwise to prejudice, the effective conduct of public affairs.”
The rationale for the application of section 36(2)(b) is as follows:
The Trust must be able to hold free and frank discussions about its services and about confidential and sensitive matters, without concern that the detail of those discussions or that advice will be prematurely disclosed. The fundamental purpose of undertaking an invited service review is to facilitate the free and frank exchange of views for the purposes of deliberation, which enables the Trust to shape and implement proposals in order to improve its services. The review report by the GGI contains analysis which reflects candid discussions between staff within the Trust and the reviewers. To be effective, invited service reviews rely on a relationship of trust and confidence between the Trust and the staff concerned. Disclosure of this report would, therefore, likely undermine this trust and inhibit the free flow of views and information that would consequently have a detrimental impact on service development and improvement.
During the review, staff were interviewed and opinions shared which forms a substantial amount of information contained in the report. This information was exchanged with the clear expectation that these discussions would remain confidential. Due to the small number of staff involved, staff could be identified by their views which would then be known by other staff, making working relationships more strained and complicated. Disclosure in this case could well exacerbate some of the problems that led to the review being commissioned in the first place. For these reasons we believe that disclosure poses a significant risk of having a chilling effect on the willingness of staff that participated in the review and the wider team from continuing to assist the Trust, and from participating in future discussions relevant to the issues involved.
The Trust must be allowed the safe space to conduct rigorous and candid reviews of its services, seek advice and deliberate openly and honestly about how to move forward without the risk of premature disclosure. There must be a safe space in circumstances such as this, which staff feel able to raise concerns and discuss issues which could benefit their work. While staff have an understanding of the FOIA, there is still an expectation that this type of free and frank discussion will remain confidential. If the information contained in the report was disclosed, staff may be reluctant to participate so freely, frankly and honestly. All levels of staff in the Directorate concerned and staff more generally, are likely to be affected by disclosure in this case which would likely have a significant chilling effect on the willingness of staff to participate in such reviews in the future. Although specific, personal identifying information can be redacted from the report under section 40(2) exemption [personal information], due to the small number of staff involved in the review the general themes and views outlined in the report could still be attributed to these staff. If staff were less willing to participate in reviews of this nature on the basis that their personal views might become known to their colleagues or the general public, this would likely undermine the ability of the Trust to effectively review its performance and implement changes to improve its services.
The rationale for the application of section 36(2)(c) is as follows:
Further to the views outlined above, the Trust’s Qualified Person is also of the opinion that disclosure of the report would otherwise prejudice the effective conduct of public affairs. The Trust is in the process of resolving some of the issues raised in the report and a safe space is necessary in which to do this. Disclosure of the report would likely prejudice the ability of the Trust to discuss and debate internally the issues it faces, the recommendations put forward and the options available to it. Although we appreciate that considerable time has passed since the review, some of the more complex issues involved remain live. The information that staff provided remains relevant to the status of the service at this time and issues are ongoing for the staff involved. Since this was an invited review and not a regulatory investigation, the willingness of staff to continue to participate and cooperate in this process is essential. Full disclosure would be likely to discourage the staff that participated in the review and those in the wider department, from continuing to assist the Trust. This would likely hinder the Trust’s ability to develop its corporate improvement plan and implement the changes that are required. Disclosure at this stage would be premature and therefore likely to prejudice the outcome of the review process.
The issues involved in the review include sensitive matters and disclosure could cause those involved distress and upset. The Trust relies on its relationship with its staff to enable free and frank communication, and has a duty to safeguard and promote positive relationships between the staff involved in such reviews and across the wider Trust. Full disclosure would likely lead to greater speculation, external comment, media attention and/or pressure from other interested parties adding prejudice to the review process. Disclosure and subsequent use of this information by others without understanding the context in which it was written, is likely to lead to sensationalised or misunderstood reporting about the service in question, which may undermine public confidence in the service and the staff that support it. The Trust requires a safe space to develop and promote its corporate improvement plan which will address the issues raised without getting unduly side-tracked with public enquiries and media attention. Disclosure at this stage would likely to prejudice the Trust’s ability to carry out its public affairs effectively and implement the necessary actions that are required to improve patient care. This would likely undermine its willingness to invite external organisations to conduct service reviews in the future.
The Public Interest Test: Section 36 is a prejudice based and qualified exemption so the Trust must apply a ‘public interest test’ and a ‘prejudice test’ to decide whether, in the circumstances of the case, the public interest in maintaining the exemption outweighs the public interest in disclosing the information.
The Trust recognises the public interest in accountability and transparency in respect of the decisions and actions it takes. Disclosure may further public understanding and public debate surrounding the issues identified by the review, what has been recommended and why, and the improvements that are required. The Trust fully appreciates the public interest in the spending of public funds and ensuring the best use of public resources. Additionally, we acknowledge the public interest in bringing to light information affecting public health and safety.
There is clearly strong public interest in ensuring the quality and safety of the Trust’s services, in demonstrating our accountability for these services and how well they are performing. We also understand how issues around quality and safety might impact on an individual basis and on the public more generally. However, this public interest is met in a variety of ways, such as through a range of internal and external quality assessment and assurance processes and CQC inspections which are formally reported. Whilst invited service review reports may also serve this purpose, it is our view that the forthcoming report that will outline the Trust’s corporate improvement plan which will address some of the issues raised by this review and will be made available via the Trust Board, is the more appropriate means by which to communicate this information to the public in these circumstances. Invited service reviews protect patient safety by supporting staff to speak up safely, which leads to robust conclusions and recommendations that are then followed up by the Trust to ensure important issues and concerns are accepted and being addressed.
It is our view that to be effective, invited service reviews rely on a relationship of trust and confidence amongst those staff involved. The Trust considers that there is a strong public
interest in respecting the confidences of those that participated in the review process and in preserving and promoting candour, reflection and freedom to speak up amongst staff. Although full disclosure of the report would provide transparency and further insight regarding the purpose of the review, we do not believe this outweighs the negative impact disclosure would likely have on future invited reviews and the willingness of staff to participate in them. Diminished cooperation by staff in this context would undermine the ability of the Trust to effectively review its performance and implement changes that would benefit patients and staff, which is firmly in the public interest. There is a public interest in safeguarding and promoting the relationships between all parties involved in these reviews, and ensuring that they remain willing to share free and frank views in the future, to help guarantee that such reviews remain fit for purpose and learning for the Trust is encouraged. It would not be in the wider interests of the public to prejudice this function.
Considering the timing of the request and the circumstances at this time, we believe the consequences of disclosure would add additional challenges for the Trust and this would not be in the public interest. Deliberations and planning which address the recommendations made in the review report remains ongoing. In order to decide on the steps and resolutions required to address the recommendations made, the Trust requires the safe space to obtain and consider free and frank internal advice and deliberate openly, candidly and honestly about how to move forward. This process needs to be rigorous so as to promote patient safety, the effective delivery of services, and to improve relationships between staff members. Disclosure of the information contained in the report at this time would be likely to prejudice this process. Those staff involved would be likely hindered or discouraged from discussing and considering the issues so openly and frankly, which would be further prejudiced by likely public enquiries and media attention. Disclosure at this time would also likely negatively impact the relationship the Trust has with staff involved in this process, thereby hindering its ability to implement the changes that are required. This would in turn compromise the Trust’s ability to assure the public that it is taking the necessary action to improve patient care. We believe such consequences would be challenging for the Trust and this would not be in the wider interests of the public. Rather it is in the best interests of the public to allow the Trust the safe space it requires to considers it options and implement the right solutions in order to address the issues identified. We believe there is compelling public interest in preserving the integrity of reviews of this nature and in giving the Trust the best opportunity to overcome the challenges it faces so that it may provide the highest quality of clinical care for its patients.
Taking into account all of these considerations, it is the view of the Trust that the public interest is best served by withholding the information contained in the report under section 36(2)(b) and (c) for the reasons explained above.
The Information Commissioner’s Office (ICO) has a duty to investigate complaints where it is
believed that an authority has failed to respond correctly to a request for information. Previous investigations and subsequent decisions by the ICO have established that it is both reasonable and in the wider public interests for NHS organisations to withhold reports which detail the findings and outcomes of Invited Service Reviews under section 36(2)(b) of the FOIA. We have considered a number of these decisions and believe the application of section 36(2) exemption is appropriate in these circumstances and this view is supported by ICO Decision Notices, reference FS50839428 and FS50730159. We have provided links to these decision notices for your information below.
Microsoft Word – FS50839428 Decision Notice for website (ico.org.uk)
Freedom of Information Act 2000 (Section 50) (ico.org.uk)
RELATED ITEMS
A Study in Delay: The National Guardian & Brighton and Sussex University Hospitals NHS Trust
Kark, Fit and Proper Persons, NHS England, Mason Fitzgerald and the Good Governance Institute
This is an article summarising BBC Panorama’s 2021 investigation about suppressed invited reviews in the NHS:
BBC 2021: Unpublished hospital patient safety reports exposed


So many bodies and public figures purporting to espouse the highest standards – when in fact they are all primarily motivated by protection of self-interests; the complaining patient or whistleblower is a low priority and hinderance to all of them. Surely it’s time for a patient led feedback and accountability – via an open an transparent platform such as TrustPilot and patient led review body that truly holds these people to account?? As all present arrangements are all inbred affairs. The recent comments of PHSO Ombudsman Rob Behrens in report, finding ‘Culture of Cover-Up in NHS’ and ‘ingrained defensiveness’ when it comes to patients suffering avoidable death . Highlighting the ‘gaping hole’ between policies aimed at improving patient safety and real-life experience on the ground with hospitals routinely failing to accept their errors. And NHS England claiming ‘we don’t recognise the term avoidable harm, avoidable death, it’s not helpful’ ; https://www.medscape.co.uk/viewarticle/ombudsman-finds-culture-cover-nhs-2023a1000eiz?ecd=wnl_ret_230706_mscpmrk-GB_mostread_etid5606564&uac=373746CR&impID=5606564&sso=true
LikeLiked by 1 person
Patient feedback arrangement – excellent idea!
As long as it doesn’t require another bolt-on, NHS-funded bureaucracy to address the ‘issues.’
LikeLiked by 1 person
This admirably documented, long-standing entanglement would have been amusing had it not been so serious that coroners and the police were involved –
“Serious concerns were raised by the local Senior Coroner over several years about the trust’s patient safety record, with recurrent reports to Prevent Future Deaths sent to the Secretary of State and NHS regulators. It was also the current Coroner who triggered the ongoing police investigation by referring cases of concern.” And – “Mr Grayson [a very busy man] is now acting as the senior trust liaison point for the police investigation into deaths at the trust.”
Good grief!
Maybe the Good Governance Institute/Co./Ltd/Inc./NGO etc. should consider inviting an independent management consultant to guide them rapidly. I’m sure the taxpaying, NHS-deficient public would be delighted.
Meanwhile, many thanks for delving into and sticking with such a depressing affair.
LikeLiked by 1 person